The U.S. Food and Drug Administration (FDA) has expanded the indication for cariprazine to include pediatric patients, approving its use for the treatment of schizophrenia in patients aged 13 years and older and for the acute treatment of manic or mixed episodes associated with bipolar I disorder in patients aged 10 years and older.
Cariprazine was previously approved for adults with schizophrenia, bipolar I disorder (manic, mixed, and depressive episodes), and as adjunctive therapy to antidepressants for major depressive disorder.
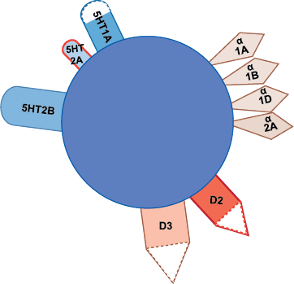
Cariprazine: Mechanism of Action
Cariprazine is an atypical antipsychotic with a distinct receptor profile. Its clinical effects are thought to be mediated through partial agonist activity at dopamine D3- and D2-receptors and serotonin 5-HT1A receptors, along with antagonist activity at serotonin 5-HT2A receptors.
Cariprazine has a higher binding affinity for D3 than D2 receptors, a feature hypothesized to contribute to effects on mood, cognition, and negative symptoms.
Cariprazine forms two active metabolites—desmethyl-cariprazine (DCAR) and didesmethyl-cariprazine (DDCAR)—which are pharmacologically equipotent to the parent compound and contribute to its prolonged clinical activity.
FDA-Approved Pediatric Indications and Dosing
According to the updated prescribing information:
| Indication |
Age Range (years) |
Starting Dose |
Recommended Dose |
Maximum Dose |
| Schizophrenia |
13–17 |
0.5 mg once daily |
1.5–4.5 mg once daily |
4.5 mg/day |
| Bipolar I Disorder (Manic or Mixed Episodes) |
10–17 |
0.5 mg once daily |
3 mg or 4.5 mg once daily |
4.5 mg/day |
Because of cariprazine’s long half-life and accumulation of active metabolites, dose adjustments should be made cautiously, with clinical response and tolerability monitored for several weeks after initiation or dose change. Initiating cariprazine is not recommended in pediatric patients while taking a strong or moderate CYP3A4 inhibitor.
Clinical Evidence in Pediatric Patients
The approval is supported by a phase I open-label study evaluating pharmacokinetics, safety, and tolerability in pediatric patients with schizophrenia or bipolar I disorder.
- Steady state achieved in 1–2 weeks for cariprazine and DCAR, and 4–5 weeks for DDCAR
- Systemic exposure increased proportionally with dose
- Minimal weight gain, cardiometabolic effects, prolactin changes, EPS
- Low suicidal ideation rates (1.4% vs 3.5% placebo)
Exploratory efficacy analyses showed improvements in PANSS scores (schizophrenia) and YMRS scores (bipolar I disorder).
Safety and Tolerability
Cariprazine was generally well tolerated. The most common treatment-emergent adverse events included:
- Sedation (18%)
- Parkinsonism (8%)
- Dystonia (8%)
- Tremor (6%)
- Blurred vision (10%)
Most adverse events were mild to moderate. No serious adverse events, discontinuations, or suicidal ideation were reported. Weight gain ≥7% occurred more frequently in younger patients (ages 10–12), highlighting the need for routine metabolic and weight monitoring.
The FDA approval of cariprazine for pediatric schizophrenia and bipolar I disorder expands treatment options for children and adolescents. Its dopamine D3-preferring mechanism, stable prolactin profile, and manageable metabolic effects may offer advantages, though clinicians should remain attentive to extrapyramidal symptoms and weight changes, particularly in younger patients.
References:
Vraylar® (cariprazine) Prescribing Information. AbbVie Inc. Revised December 2025. vraylar_pi.
Riccobene T et al. J Child Adolesc Psychopharmacol. 2022;32(8):434–443.Abstract
Additional Education and Resources:

Patient Education Disorder Guides
Medication Guides: Cariprazine