The U.S. Food and Drug Administration (FDA) has approved milsaperidone (Bysanti™) for the acute treatment of manic or mixed episodes associated with bipolar I disorder and for the treatment of schizophrenia in adults. Milsaperidone is a novel atypical antipsychotic and the major active metabolite of iloperidone.
The approval was supported by a comprehensive clinical development program that leveraged established efficacy and safety data for iloperidone, along with pharmacokinetic studies demonstrating bioequivalence between milsaperidone and iloperidone across the full therapeutic dose range, following both single-dose and multiple-dose administration.
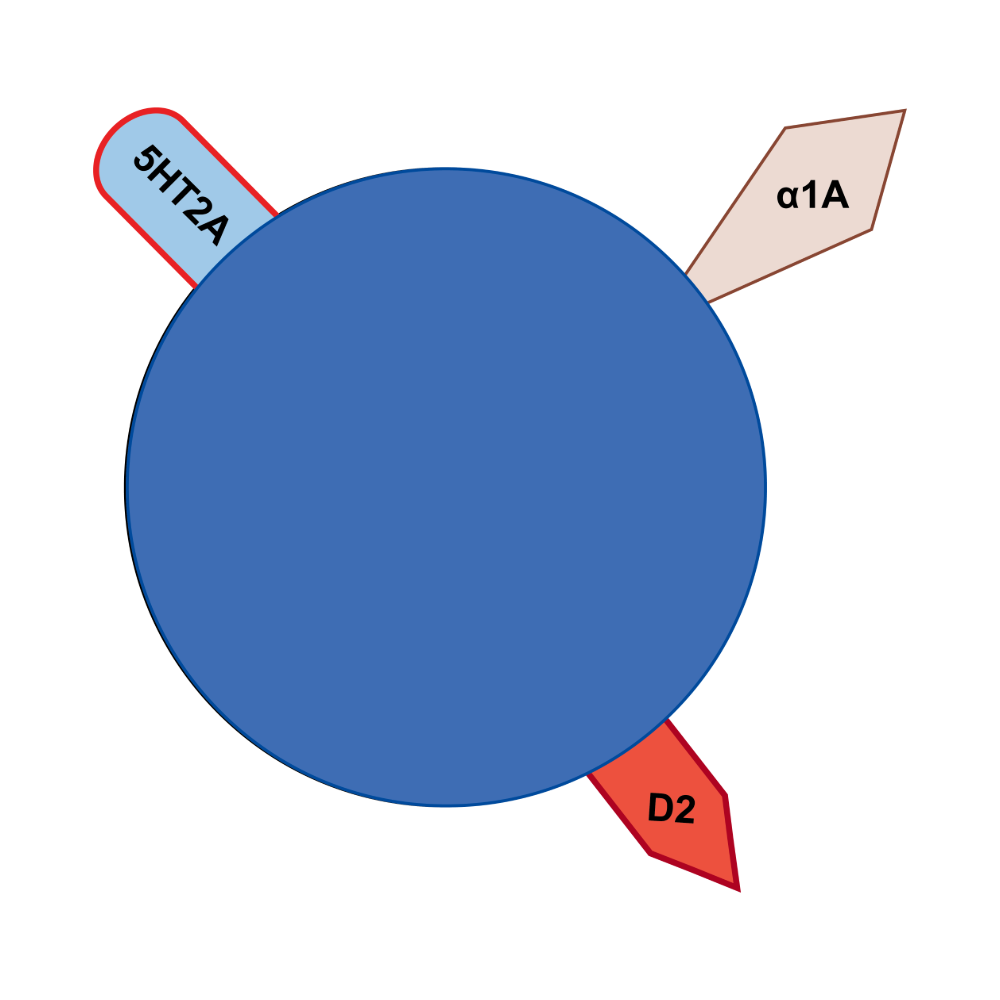
Milsaperidone: Mechanism of Action
Milsaperidone exerts antipsychotic and antimanic effects through dopamine D2 receptor antagonism and serotonin 5-HT2A receptor antagonism, with additional high-affinity a1-adrenergic receptor antagonism.
This receptor profile, shared with iloperidone, underlies its antipsychotic and antimanic efficacy, low rates of extrapyramidal symptoms and akathisia, and a distinct tolerability profile characterized by orthostatic effects during titration rather than dopamine mediated motor adverse effects. As a new chemical entity that rapidly interconverts to iloperidone, it provides dual active molecules that work in tandem by antagonizing dopamine D2, serotonin 5-HT2A, and alpha1 adrenergic receptors to modulate key pathways involved in these disorders.
Milsaperidone readily crosses the blood–brain barrier and interconverts with iloperidone in vivo, supporting its classification as a new chemical entity with established central nervous system exposure and clinical relevance
Schizophrenia: Efficacy and Safety
The efficacy of milsaperidone in schizophrenia is supported by multiple randomized, double-blind, placebo-controlled and active-controlled trials of iloperidone in adults with acute schizophrenia. In a pivotal 4-week study (NCT00254202), iloperidone produced statistically significant reductions in Positive and Negative Syndrome Scale (PANSS) total scores compared with placebo and demonstrated efficacy comparable to ziprasidone.
Across short-term and long-term studies, iloperidone was associated with:
- Significant improvements in positive and negative symptoms
- Relapse prevention in stabilized patients
- Low rates of EPS, akathisia, and prolactin elevation
- Minimal metabolic laboratory changes
These findings form the basis for extrapolating schizophrenia efficacy and safety to milsaperidone under FDA bioequivalence guidance.
Bipolar I Disorder: Acute Mania and Mixed Episodes
Approval for acute bipolar I disorder (manic or mixed episodes) was supported by a phase 3, randomized, double-blind, placebo-controlled trial of iloperidone (NCT04819776) in adults hospitalized for acute mania. Iloperidone demonstrated statistically significant improvement in Young Mania Rating Scale (YMRS) total scores compared with placebo at week 4 (least-squares mean difference -4.0; P < .00001).
Secondary outcomes showed improvement in Clinical Global Impressions–Severity (CGI-S), Clinical Global Impressions–Change (CGI-C), and YMRS response and remission rates. A post hoc item-level analysis also demonstrated significant improvement on the YMRS sleep item, suggesting benefit for both reduced sleep duration and decreased perceived need for sleep—core features of manic episodes.
The safety profile in bipolar mania was consistent with prior schizophrenia studies, with low rates of akathisia and EPS and no new safety signals identified.
Pharmacokinetics and Dosing Considerations
Single-dose and steady-state studies confirmed that milsaperidone and iloperidone are bioequivalent with respect to Cmax and AUC, meeting FDA criteria for bioequivalence (90% CI within 80–125%). Interconversion between the two compounds occurs across the approved dosing range.
As with iloperidone, gradual titration is recommended to mitigate orthostatic hypotension related to a1-adrenergic antagonism. Unlike iloperidone, milsaperidone does not rely on CYP2D6 for activation; however, CYP2D6 still contributes to its clearance and overall exposure, so dose adjustments remain necessary in poor metabolizers or with strong CYP2D6 inhibitors.
Safety and Tolerability
Across the clinical development program, the most commonly reported adverse events were dizziness, orthostatic hypotension, primarily during titration, dry mouth, nasal congestion, and somnolence. Rates of extrapyramidal symptoms, akathisia, and prolactin elevation were low and comparable to placebo in bipolar studies, and lower than many comparator antipsychotics in schizophrenia trials. Although QTc prolongation was observed, it remained within a predictable and manageable range with appropriate dosing and avoidance of contraindicated medications.
Clinical Perspective
Milsaperidone expands the treatment landscape for both schizophrenia and bipolar I disorder by offering:
- Proven efficacy across psychotic and manic symptom domains
- A favorable motor tolerability profile
- A mechanistically familiar but clinically differentiated option within the atypical antipsychotic class
Its approval also represents a translational regulatory strategy, leveraging bioequivalence to efficiently bring a new chemical entity to clinical practice while maintaining a well-characterized benefit–risk profile.
Additional Education and Resources:
References:
Torres R et al. Efficacy and Safety of Iloperidone in Bipolar Mania. J Clin Psychiatry 2024
Chadwick SR et al. Pharmacokinetic Results of Single-Dose and Multiple-Dose Bioequivalence Studies of Milsaperidone and Iloperidone. ASCP 2025